JAPANESE ENCEPHALITIS – General Characteristics, Transmission and Epidemiology, Pathogenesis and Pathology, Clinical Features, Laboratory Diagnosis, Treatment and Prevention
Japanese encephalitis (JE), a severe mosquito-borne infection of the CNS, is a leading cause of childhood encephalitis in Asia.
GENERAL CHARACTERISTICS
Etiology and History
The causative flavivirus is related antigenically to West Nile virus. The virus was first isolated from human brain of an epidemic case in Japan in 1924 and its transmission by mosquito was proved in 1936. It is an RNA virus. This disease has been reported from several countries in both tropical and temperate zones.
Transmission and Epidemiology
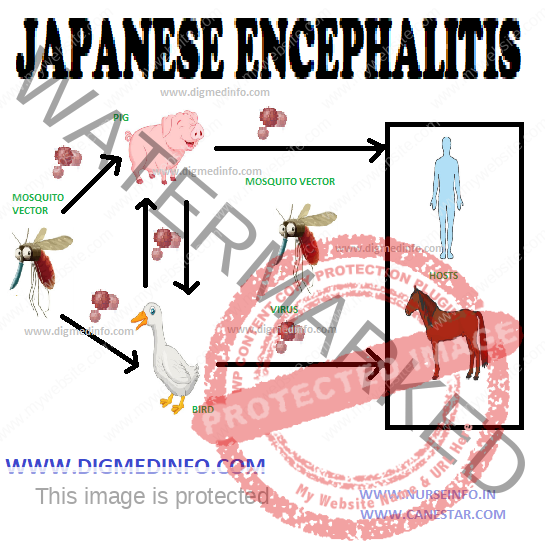
The virus exists in reservoir hosts and man is affected from them. Pigs, birds such as pond herons, cattle-egrets, bats, buffaloes, and cattle harbour the virus. In India, pigs constitute the major reservoir in which the virus multiples. Migration of birds accounts for the regional spread of infection. Though the virus is seen in several animals, it is pathogenic only to man and a few other mammals like horses. Poverty, unsatisfactory dwelling conditions and co-existance with cattle, birds and pigs facilitate transmission.
The vector Culicine mosquitoes act as the main vectors. Important among them are Culex tritaeniorhyncus, C.pseudovishnui and C. vishnui. Anopheles barbirostris can also transmit this virus.
The natural transmission cycle of the virus is birds → mosquito → bird and pig → mosquito → pig. Human infection occurs only when the mosquito population increases and man – mosquito contact is established. Risk for infection is highest in rural locations where rice fields, pigs and humans coexist.
In the vast majority, the infection remains subclinical. The ratio of overt to inapparent infection varies from 1:300 to 1:1000. Children below 15 years suffer more. The peak incidence is between 2 to 10 years and males suffer more.
Pathogenesis and Pathology
After an infective bite by the mosquito, the virus propagates locally and in the regional lymph nodes. Viremia leads to affection of several organs mainly the brain unless the infection is modulated by prompt immune response. Neuroinvasion probably occurs as the virus grows through vascular endothelial cells to the parenchymal side. The virus enters the neurons and leads to widespread degenerative and necrotic changes. Most marked lesions occur in the cerebral cortex, thalamic nuclei, corpus striatum, and brainstem with relative sparing of the white matter. Cerebellar cortex and spinal motor neurons may also be affected, but the affection is less marked. There is occlusion of the smaller arterioles leading to extensive focal ischemia and necrosis. Perivascular hemorrhages and mononuclear infiltration are seen.
CLINICAL FEATURES
The incubation period ranges from 5-15 days. Three stages are recognizable- prodrome, acute encephalitis and convalescence.
Prodromal stage:
The onset may be gradual (4-5 days) or acute (12-24 hrs) or abrupt (1-6 hrs). The disease starts as fever with chills, headache, meningism, convulsions, psychotic behaviour and coma. Majority of cases recover in 4-5 days and clinical diagnosis can be made initially, only during epidemics.
Acute encephalitic stage:
The fever persists at 40-41o C, the pulse is rapid and neurological manifestations predominate. Symptoms such as convulsions (70%), altered sensorium (90%), focal neurological deficits, signs of meningeal irritation (30%) and coma supervene. Supranuclear ocular palsies are common. Cranial nerve palsies and papilledema are uncommon. These features help to identify JE from tuberculous meningitis. Rapid onset of paralysis such as hemiplegia or monoplegia is characteristic. Plantar responses are bilaterally extensor.
Other manifestations of brain involvement include cerebellar signs, extrapyramidal signs and dystonic postures.
Convalescence:
Neurological function is regained gradually over several weeks, most of them by 6-12 weeks. For the rest further recovery occurs after discharge over intervals of months to years.
Laboratory Findings and Diagnosis
Leukocytosis may be present and liver enzymes may be mildly elevated. CSF shows lymphocytic pleocytosis. The cell count varies from 10-1000 cells/cmm, with an average of 100-200 cells. CSF protein may be raised to 50-250 mg/dL; sugar is usually normal. EEG abnormalities are present in the acute phase. These include a pattern of diffuse delta wave activity and rarely, spike and wave discharges. EEG findings are not helpful in predicting the outcome. ECG may show nonspecific ST-T changes indicating myocarditis.
MRI reveals diffuse white matter edema and abnormal signals mainly in the thalamus, often with evidence of hemorrhage in the basal ganglia, cerebellum, midbrain, pons and spinal cord. The clinical diagnosis is easy during epidemics. Since this disease often occurs sporadically, JE should always be considered in cases of encephalitis.
LABORATORY DIAGNOSIS
Laboratory diagnosis in most cases depends principally on the serologic testing of the serum and also CSF by antibody capture enzyme linked immunosorbent assay (ELISA). IgM and IgG antibodies can also be detected by indirect immunofluorescence assay. The assay is more than 95% sensitive when serum specimens are tested 7 to 10 days after the onset. Hemagglutination inhibition (HI) or complement fixing antibodies can be demonstrated in paired sera and these give evidence of infection in retrospect. Neutralizing antibody titer of 80 in the serum is suggestive. Fourfold rise in titer is confirmatory. CSF neutralizing antibody titer of 10 is confirmatory.
The virus can be isolated from blood only during the first few days of illness, usually preceding the onset of neurologic symptoms. Isolation of the virus from the CSF indicates the absence of protective antibodies and therefore, poor prognosis.
TREATMENT AND PREVENTION
Treatment
There is no specific treatment. Supportive treatment includes the correction of fluid and electrolyte abnormalities, measures to control cerebral edema, anticonvulsants and maintenance of nutrition. Use of human immunoglobulin in a dose of 150-200 mg/kg bw early in the disease has been claimed to reduce the severity and mortality of the disease. There are preliminary data on the possible effectiveness of alpha-interferon for treatment of JE. Further studies are required to confirm the effect.
PREVENTION
Killed vaccine using Nakayama Yoken strain of the virus is available for prophylaxis. It is a formalin-inactivated vaccine. It is supplied as freeze dried vaccine, which has to be stored below 10oC. The reconstituted vaccine should be used within eight hours if kept cold. It should not be frozen. The recommended schedule for adults in JE endemic areas is two doses of 1 mL each given SC at an interval of 1-2 weeks. Protection starts one month after second dose. A booster dose should be given after one month but within one year. One more booster should be given after three years for full protection. In nonendemic areas repeated booster doses should be given every three years.
For children below three years the dose of vaccine is 0.5 mL, other conditions remaining the same. The vaccine is immunogenic, and safe with only rare side effects. A live attenuated cell culture derived vaccine (SA14 14-2 strain) has been used safely and with high effectiveness in China.
Anti-mosquito measures help to limit the spread. Travelers to endemic areas should protect themselves against mosquito bite.